TRAEC1.0 Strategy Theoretical Framework and Assessment Process
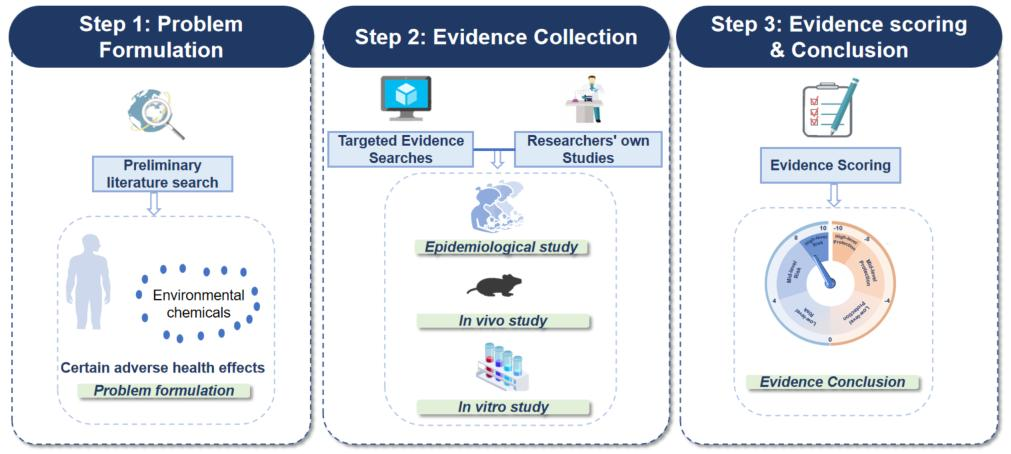
1. The theoretical framework of the TRAEC strategy consists of three parts:
(a) Step 1: Problem Formulation
Researchers can select target environmental chemicals based on their interests and experience and determine health effects according to their research fields. It is recommended that researchers first prepare a simple plan, including database searches, keywords, and inclusion/exclusion criteria, to conduct a systematic literature search for evidence of the health impacts of the target chemicals within the specified scope.
At the start of the risk assessment, a scientific question should be posed to guide the entire research process. For assessments with specific research objectives, the target chemicals and health outcomes can be directly determined. For cases without specific research objectives, the target health outcomes are the only undetermined component. Among all possible health impacts found in the search, researchers should select the health effects they are most interested in as the target outcomes based on the research findings.
(b) Step 2: Evidence Collection
Risk assessment of environmental chemicals requires a comprehensive evaluation of existing research evidence, including targeted evidence searches and researchers’ own experiments. By integrating evidence from different sources, such as databases, websites, and laboratories, specific data on epidemiological, in vivo, and in vitro studies can be obtained.
Epidemiological Studies: Since risk assessment is aimed at human health, it must include epidemiological data on human health to ensure the completeness of the evidence chain. Researchers must first identify the target population and then assess the internal/external exposure to the target chemicals, such as exposure routes and doses. This also includes baseline information about the study population, such as personal identity, lifestyle, disease history, treatment status, and reproductive history. Methods for exposure assessment can include prospective follow-up and cross-sectional surveys; in some cases, retrospective survey methods, such as standardized questionnaires completed online or by phone, can also be used. Additionally, to assess and quantify adverse health effects related to target chemicals in the population, researchers can investigate incidence rates under the corresponding exposure conditions through literature and public databases. If relevant studies are not found, researchers will collect epidemiological data to assess the dose-response/effect relationship in the population.
In Vitro Studies: Researchers need to review literature to examine results from published in vitro studies. Additionally, for mechanisms that are not yet elucidated, researchers can conduct their own in vitro studies to verify and supplement literature evidence, including molecular initiating events, cellular responses, or cellular phenotypes of the target chemicals. This can also provide new mechanistic evidence to enrich the existing evidence base.
In Vivo Studies: Similar to the design of in vitro studies, these are used to address mechanisms not yet elucidated in existing in vivo research. Researchers can conduct in vivo experiments to verify and supplement organ responses and biological reactions to exposure to target chemicals. In principle, redoing homogeneous animal experiments should be avoided.
(c) Step 3: Evidence Scoring & Conclusion
Risk assessment of environmental chemicals requires a comprehensive evaluation of existing research evidence, including epidemiological, in vivo, and in vitro studies. It is challenging for researchers to systematically, transparently, and consistently evaluate comprehensive evidence to obtain highly reliable conclusions. Therefore, the development of "Comprehensive Evidence Scoring Rules" is intended to address these challenges.
Data sources are diverse, including databases, authoritative institutions, and researchers’ laboratories. This theoretical framework is used to assess health risks by integrating four key components: reliability scores, concentration weights, relevance scores, and completeness scores. The scores of different types of evidence are combined, and the overall validity, direction, and strength of the association are derived based on scientific calculation formulas.
All evidence should be independently scored by two researchers, with the average score being the final score for each item. If the scores differ by 2 points or more, another team member should arbitrate. If a study includes any two or more types of evidence, i.e., epidemiological/in vivo/in vitro evidence, researchers need to summarize the scores for these two or all types of evidence.
TRAEC Strategy Evaluation Process
Step 1: Problem Formulation
1.1. Focus on environmental exposure factors that can be accurately measured in the environment and population, have a high detection rate, and are currently uncontrolled, such as pesticides, perfluorochemicals, heavy metals, etc.
1.2. Pay attention to adverse health outcomes with high current incidence, reliable measurement results, and potential health risks to the human body, such as COPD, diabetes, obesity, fatty liver disease, occupational diseases, etc.
1.3. Construct a scientific problem framework for the potential health risks of specific chemicals, such as “What is the relationship between prenatal atrazine exposure and neurodevelopment in offspring?”
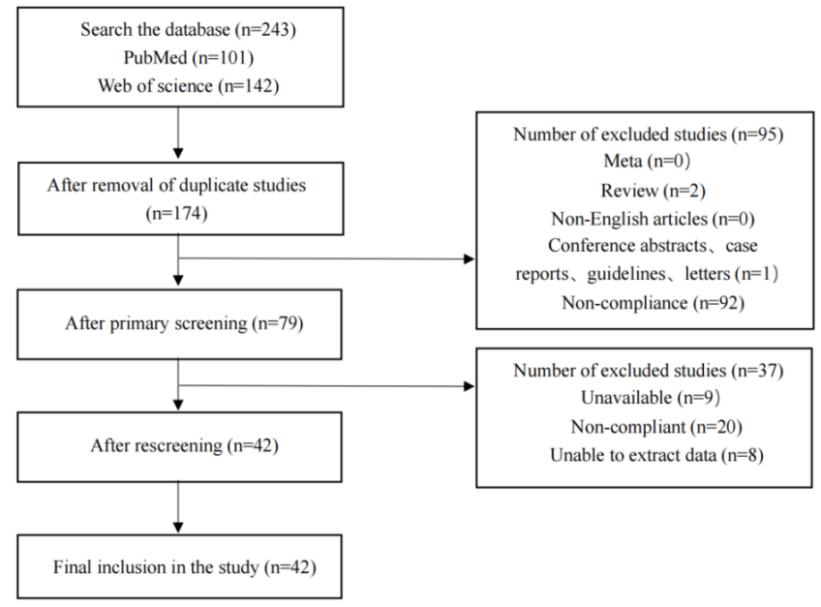
Step 2: Evidence Collection
2.1. Evidence collection includes both existing and newly conducted evidence, and comprehensively integrates various types of research data. This is the major difference from traditional risk assessments.
2.2. Researchers need to review existing studies, including published epidemiological, in vivo, and in vitro research results. Integrate data from various sources (such as literature databases, authoritative institutions, and official websites) to obtain evidence.
(1) Determine Evidence Selection Criteria:
Define the types of evidence to be collected: epidemiological, in vivo research, in vitro research, etc.
Define the content of evidence collection: gather global research data on the subject.
Define the time range for evidence collection.
(2) Specify Evidence Retrieval Strategy
Identify search databases (e.g., PubMed, Web of Science, ScienceDirect, etc.).
Define keywords for exposure and outcomes (using MeSH to determine subject terms, subheadings, free terms).
Develop relevant search strategies for all databases.
(3) Retrieve Relevant Evidence:
Conduct independent searches by two people, screen and summarize evidence to ensure no bias in the selection process.
Discuss with a third high-level researcher if disagreements arise.
(4) Include and Exclude Research Evidence:
Exclude irrelevant content during initial screening based on abstracts and categories, such as Meta-analyses, Reviews, Conference abstracts, Case reports, Guidelines, etc.
During detailed screening, read the full text of the literature, and exclude content from which relevant data cannot be extracted or is irrelevant,for example:
(5) Summarize All Research Results:
Organize and analyze all research results according to study types and present them clearly. They should at least include: literature code, study subjects, exposure or detection methods, research results, research conclusions, effect concentrations, for example:
Epidemiological Studies:
| PMID | Experimental Subject | Exposure Mode | Research Results | Conclusion | Concentration |
| 36809709 | 710 pregnant women in the second trimester were randomly recruited | A total of 49 pesticides were measured simultaneously using gas chromatography-triple quadrupole tandem mass spectrometry (GC–MS/MS). They assessed neuropsychological development in children using the Ages and Stages Questionnaire (ASQ), Third Edition. | Prenatal exposure to pesticides might adversely alter child neurodevelopment. | Developmental neurotoxicity associated with prenatal exposure to pesticide | Atrazine (serum) LOD:0.05ng/ml GMD:1.04ng/ml |
In Vitro Studies:
| PMID | Experimental Subject | Exposure Mode | Research Results | Research Results | Concentration |
| 28027470 | Male quail chicks (Coturnix C. coturnix), aged 18 days with an average weight of 86.7 ± 6.4 g | 50 mg/kg, 250 mg/kg and 500 mg/kg atrazine were administered once a day orally by gavage for 45 days. | Atrazine enhanced the activities of cerebellar CYP450s and increased the contents of CYP450 in quails. The cerebellar PXR/CAR pathway responses were activated by exposing to atrazine. | Atrazine exposure induced cerebellar toxicity. | 50, 250, 500 mg/kg |
In Vivo Studies:
| PMID | Experimental Subject | Exposure Mode | Research Results | Research Results | Concentration |
| 34146662 | hESC-H9 cell line (WA09, WiCell Research Institute, USA) | NSCs were exposed to 100 μM atrazine, starting from 1 DIV for either 5, 10 or 15 days (DIV 5, 10, 15), total medium change containing 100 μM atrazine was performed twice a week. | The dopaminergic system was not the only target of atrazine neurotoxicity, glutamatergic neurons and astrocytes were also adversely affected. | Atrazine not only inhibited proliferation and induced cell cycle arrest but curbed early neural differentiation.. | 300, 500 μM |
Investigate Unclear Mechanisms
For mechanisms that are not well-defined, researchers can conduct epidemiological, in vivo, and in vitro studies to verify and supplement literature evidence, including the body's response to exposure to target chemicals, molecular initiating events, cellular responses, or cellular phenotypes.
Main Difference from Traditional Risk Assessment
Unlike traditional risk assessment, which does not include newly conducted research, the TRAEC strategy allows and encourages the inclusion of new research.
Step 3: Evidence Scoring & Conclusion
Risk assessment of environmental chemicals requires a comprehensive evaluation of existing research evidence, including epidemiological, in vivo, and in vitro studies. For individual researchers, systematically, transparently, and consistently assessing the full range of evidence to derive high-reliability conclusions remains challenging. Therefore, the development of a "Comprehensive Evidence Scoring System" is designed to address these challenges.
Data sources are diverse, including databases, authoritative institutions, and researchers' laboratories. This framework evaluates health risks by integrating four key components: reliability score, concentration weight, relevance score, and completeness score. The scores from different pieces of evidence are summed according to a formula to determine the overall validity of the evidence, the direction, and strength of the associations.
Scoring system
The TRAEC strategy's scoring system is divided into four parts:
Part1: Reliability Scores
Part2: Weight of Concentrations
Part3: Correlation Scores
Part4: Integrity Scores
1. Reliability Scores
Reliability assesses the quality of study design and implementation. It scores the reliability of epidemiological, in vivo, and in vitro research designs and implementations. In the descriptions below, these can be considered as 0 points (no relevant conditions), 0.5 points (partially meets conditions), and 1 point (meets all conditions).
Epidemiological studies
In vivo studies
In vitro studies
1.1 Epidemiological studies
| Participant and sample size |
| 1.Is the characteristics of the participant sufficient, such as age, gender, BMI, etc? |
| 2. ...... |
| Study design |
3. Is the chosen study design appropriate for obtaining the specific data on the substance? For example, the consideration of the selected exposure population, exposure time, and pattern. |
4. ...... |
| Data collection and analysis |
| 5. Is the detection or assessment of exposure assessment scientifically appropriate? |
| 6. ...... |
| Plausibility of data |
7. Are the statistical methods for data analysis provided and applied in an appropriate, transparent manner? |
| 8. ...... |
| Bias |
9. Baseline data for both included and excluded populations, as well as high and low exposure groups, are well-balanced, comparable, and adjusted for significant confounding factors. |
10. ...... |
1.2 In vivo studies
| Test compound |
| 1. Is the chemical name, source and the purity, of the test compound given? |
2. ...... |
| Animal model |
3. Is the animal model used for investigating the test compound and selected endpoints reliable and sensitive? |
| 4. ...... |
| Dosing and administration of the test compound |
5. Is there a reasonable exposure mode stated? For example, it includes a clear explanation of duration, dosage, and routes of exposure, etc.. |
6. ...... |
| Data collection and analysis |
7. Are the test and/or analytical methods used sufficiently described to allow for evaluation of the reliability of results. |
| 8. ...... |
| Bias |
| 9. Are experimental conditions consistent across all study groups? |
| 10. ...... |
1.3 In vitro studies
| Test compound |
| 1. Is the chemical name, source and the purity, of the test compound given? |
2. ...... |
| Test System |
3. Is there a dependable and sensitive test system (e.g., cell line/cells /tissue /organ/ embryo/sub-cellular fractions) with metabolic capability, where applicable, utilized to investigate the test compound and endpoints? |
4. ...... |
| Administration of the test compound |
5. Are the duration of exposure and the concentrations used suitable for the test system and investigated endpoints? |
6. ...... |
| Data collection and analysis |
7. Are reliable and sensitive tests and/or analytical methods used for investigating the endpoints? |
8. ...... |
| Bias |
| 9. Are experimental conditions consistent across all study groups? |
| 10. ...... |
2. Concentration Weight
The working concentration of a substance is an important factor affecting its toxic effects. Most of the time, we do not wish to base toxicological evidence on high-concentration exposures. In practice, there can be significant differences in the concentrations of target chemicals across different studies. Therefore, we need to establish a concentration-based adjustment weight.:
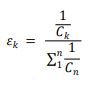
It is important to note that exposure concentrations vary in different types of studies and exposure methods, and these have various forms of description, for example:
In epidemiological studies, exposure assessments based on biological samples are considered internal exposure concentrations. Exposure concentrations based on serum/plasma/whole blood should be converted to those based on urine, hair, etc.
In in vivo studies, oral or drinking water exposure in rodent models is considered external exposure concentration, while injection exposure is considered internal exposure concentration. Additionally, exposure concentrations should be converted across different species.
In in vitro studies, cell exposure based on culture media is typically considered internal exposure concentration, and molar concentration should be converted to mass concentration.
3. Correlation Scores
Correlation reflects the direction of association between exposure and outcome and is divided into positive and negative correlations.
Type of study | Score | Associate Direction |
Epidemiological studies/In vivo studies/In vitro studies | 1 | Positive correlation |
| 0 | No significant correlation | |
| -1 | Negative correlation |
4.Integrity Scores
Completeness refers to the degree to which all types of evidence are combined, including epidemiology, in vivo and in vitro experiments.
| Type of study | Grading Criteria | Score |
| Epidemiological studies/In vivo studies/In vitro studies | Contain any of them | *1/3 |
| Contain two of them | *1/2 | |
| Contain none of them | *1 |
5. comprehensive evidence score
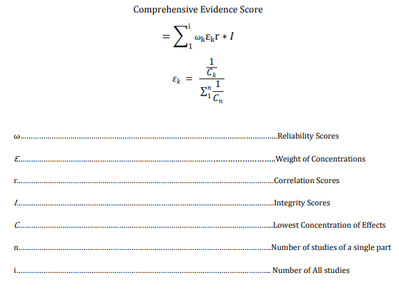
6. Risk Thresholds
The comprehensive evidence scores provide a transparent method for integrating complex research results into quantitative scores, which serve as the basis for supporting conclusions. We categorize the health risk of target chemicals into low, medium, or high levels: (0, 4] is low-level risk, (4, 8] is medium-level risk, and (8, 10] is high-level risk. Similarly, protection levels are categorized as follows: (0, -4] is low-level protection, (-4, -8] is medium-level protection, and (-8, -10] is high-level protection.


